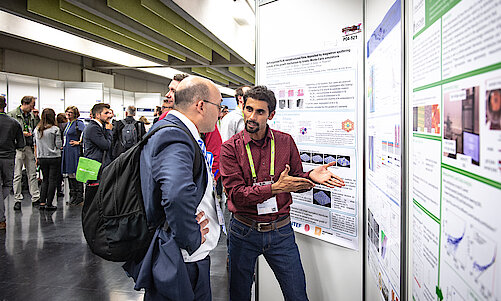
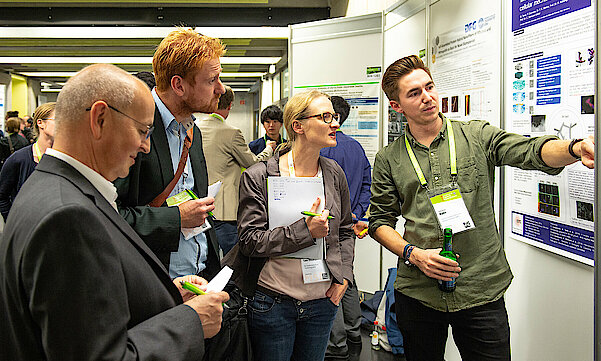
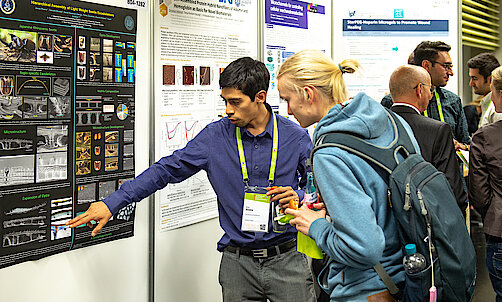
Do not miss the Poster Session at MSE 2024 on the first day of the conference. Network with colleagues and up-and-coming talent and learn about the latest research in an informal atmosphere. Share your knowledge or get feedback from industry and research experts.
The best poster of each topic will be awarded. The winners will be announced at the closing ceremony.



MSE 2024
24 - 26 September 2024 | Hybrid Congress in Darmstadt (Germany) & Online
MSE 2024
24 - 26 September 2024 | Hybrid Congress in Darmstadt (Germany) & Online
Subscribe to our newsletter for regular updates about materials science topics!
After subscribing, you will receive an email from us with a confirmation
link.
Only after clicking this link your registration is completed.